05-Nov-2025
The fungal tRNA ligase (TRL1) is essential for tRNA repair, as well as splicing mRNA in the unfolded protein response (UPR) brought about by stresses on the endoplasmic reticulum. Following several recent crystallographic studies on the N-terminal adenylyl transferase domain (LIG) of TRL1 in Chaetomium thermophilum (CHTHE), as well as the GTP-dependent kinase domain (KIN) in Candida albicans (CAGLA). This study from McEwen et al (2025), analysed the ATP-binding LIG domain in C. albicans, utilising crystallisation sample preparation techniques at IGBMC (part of Instruct-FR) through Instruct-ERIC access.
The aim of the study was to identify whether TLR1 could be an effective antimicrobial target for drug development. Its fundamental nature in the activity of fungi make it an attractive prospect. The first step was to assess conservation of the domain across other Candida species, and other fungi. LIG domains from C. glabrata revealed sequence 35%–36% similarity with C. thermophilum (20%) and Saccharomyces cerevisiae (36%), with particular conservation of the ATP-binding domain. This similarity ensured that CHTHE could be used as a base for molecular replacement – essential in minimising phase problems with solving the structure from crystallographic data.
The crystal structure of the LIG domain in CAGLA identified two promoters in its asymmetric unit, named Molecule A (MolA) and Molecule B (MolB). These molecules share a highly similar overall fold, but with small movements and variability in the subdomains. MolB represents an open conformation, whilst MolA is closed. The conformation of both protomers is maintained by interactions of several sites, primarily Switch 2 (SW2) and Switch 1 (SW1). These sites are loops that connect various β-strands, which help to influence and order the confirmational shape of the LIG domain. The next step, however, was to understand whether ligand binding was integral to the conformational changes between the protomers, solving them in open or closed configurations.
Electron density maps showed that a single AMP molecule occupied the ligand binding site of each protomer, despite being incubated with ATP. This indicates that ATP was hydrolysed to AMP during crystal growth. The AMP in closed state MolA forms a conformation that promotes stability and structural function – the AMP becomes “caged: by the surrounding protein structure – forming strong bonds with several residues across the binding domain.
In the open confirmation MolB, the AMP phosphate group forms hydrogen bonds and salt bridges with various sites, again stabilising the complex in its configuration.
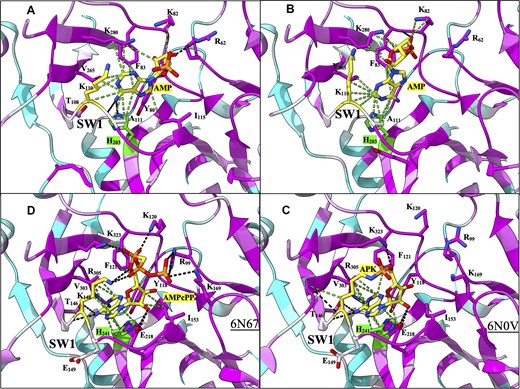
Figure 1. Structural comparison of the ATP binding pocket of Rnl6 ligases LIG domain. Atomic contacts are represented by dashed lines: hydrogen bonds are colored in black, van der Waals interactions in green. The color of the residue ranges from cyan (less conserved) to purple (more conserved). Carbon atoms are represented in purple, oxygen atoms in red, nitrogen atoms in blue, and phosphorus atoms in orange. Carbon atoms are illustrated in yellow for the lysine residue that becomes covalently bonded to AMP during the ligation. In green are the carbon atoms of the histidine residue described as the “gatekeeper”. (A) The AMP molecule complexed with CAGLA LIG in its “open” state (molA). AMP, shown as sticks, in the intermediatesyn conformation. (B) The AMP molecule complexed with CAGLA LIG in its “closed” state (molB). AMP, shown as sticks, in the anti conformation. (C) The APK intermediate of the CHTHE LIG complex (PDB ID: 6N0V). (D) The Michaelis–Menten AMPcPP complex of the CHTHE LIG complex (PDB ID: 6N67).
Figure 1 illustrates the key residues and sites in which both protomers are bound to the AMP ligand in CAGLA, as well as in the comparative protein in CHTHE. By solving and reporting the key sites and residues that are fundamental to the binding and therefore function of the TRL1 ATP-binding domain, it “lays the groundwork for the development and optimisation of specific inhibitors or agonists” against fungal infection.
Group lead Giuseppe D Tocchini-Valentini commented, "Instruct-ERIC has been a great boost to our project, providing tools and expertise that are not easily available elsewhere. It facilitated smooth access to advanced structural biology technologies, and this support improved our research quality and speed, especially in important areas like infectious diseases. Overall, it enriched our work, fostered collaboration, and opened new opportunities for discovery. This experience truly highlights the power of shared resources and collaboration in science."
The study provides targets for drug development that prevent effective TRL1 function, whilst minimising adverse effects on the host cell.